R&D Programs
Discovery
ADME / PoC
IND Enabling
Phase 1
Phase 2
CB-103 CSL-NICD
CB-103 CSL-NICD
NextGen CSL-NICD
Virus Induced Cancers
AML – Oncology
MYB – Oncology
R&D Programs
Discovery
ADME / PoC
IND Enabling
Phase 1
Phase 2
CB-103 CSL-NICD
CB-103 CSL-NICD
NextGen CSL-NICD
Virus Induced Cancers
AML – Oncology
MYB – Oncology
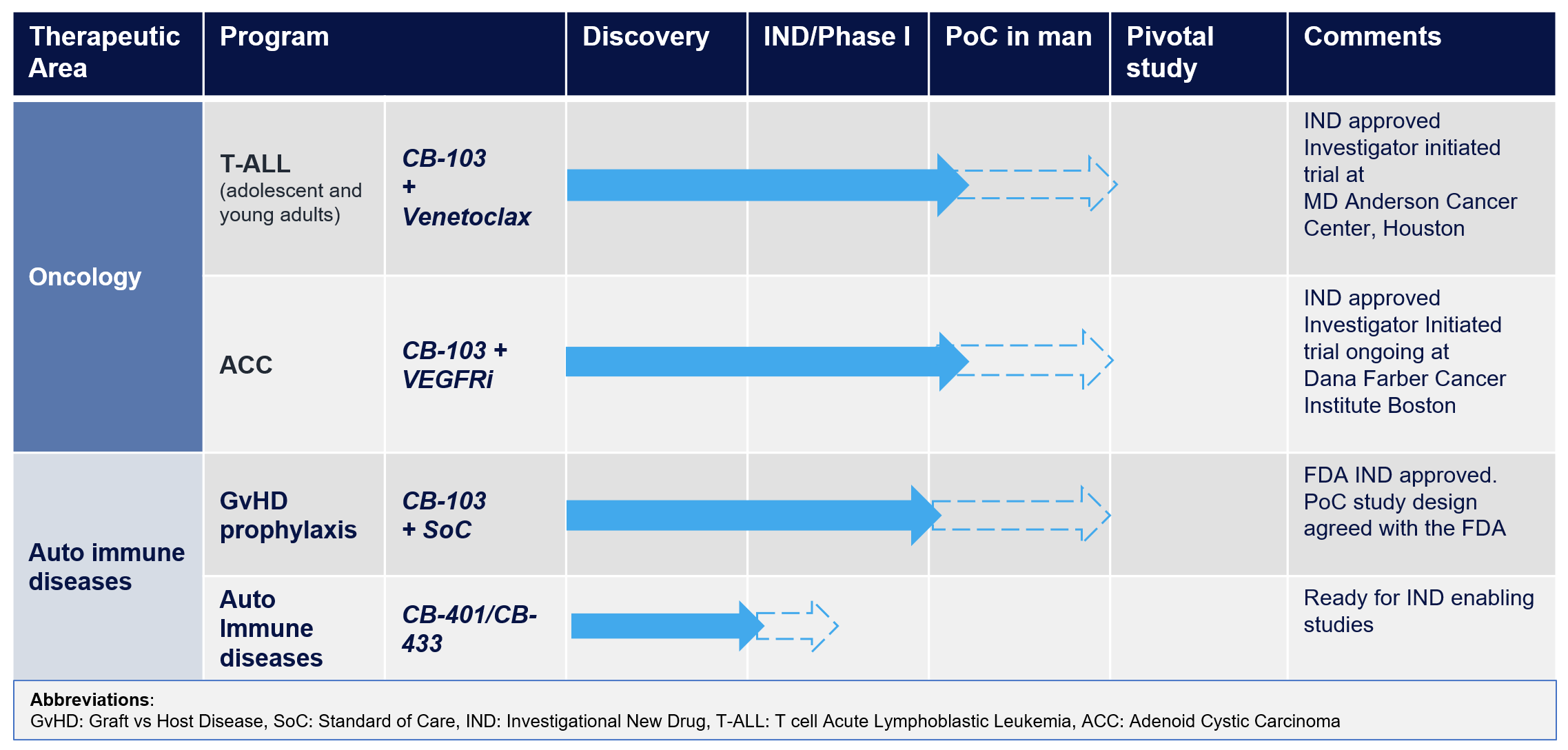
The outstanding potency and safety of CB-103 has been confirmed in the Phase 1 clinical trial, showing strong target engagement and downregulation of key target genes. CB-103 has also demonstrated strong clinical benefit for ACC patients achieving long-term disease control. Achieving clinical proof of concept for CB-103 is the foundation to now enter Phase 2 clinical development with treatment of patients with ACC, T-ALL, breast cancer and rare NOTCH positive tumors.
In addition, the company has a rich pipeline of pre-clinical assets, with in vivo proof of concept in relevant animal models, covering a wide range of indications such as oncology, virus-induced cancers, and autoimmune and inflammatory disorders. These achievements put Cellestia on an excellent track to bring first-in-class innovative therapies to patients, addressing currently unmet medical needs across a wide range of indications.

The Phase 1 clinical trial of CB-103 has confirmed its exceptional safety and biological activity, as evidenced by its robust engagement with the intended targets and effective downregulation of relevant target genes. The successful completion of the phase I study establishes a strong foundation for advancing CB-103 to Phase 2 clinical development. This next phase will focus on treating genetically defined orphan cancer indications such as T-cell acute lymphoblastic leukemia (T-ALL) and adenoid cystic carcinoma (ACC).
With its confirmed safety profile and compelling scientific rationale, CB-103 is also being developed for the prevention of graft-versus-host disease (GvHD) in patients undergoing allogeneic hematopoietic stem cell transplant.
Furthermore, Cellestia is making significant progress in its promising pipeline of pre-clinical assets. These assets have undergone in vivo testing in relevant animal models, providing compelling proof of concept for the treatment of chronic autoimmune and inflammatory disorders.
CB-103: a new mode of action
Innovative Approach
Cellestia Biotech is developing drugs against cellular signaling with unambiguous role in human cancers and auto immune diseases. However, instead of targeting extracellular or cytoplasmic proteins, we are targeting specific nuclear transcription factors downstream of these pathways.
Using a combination of structural biology, structure activity relationship and machine learning, Cellestia has been successful in targeting and advancing transcription factor inhibitors into clinics.
The approach pursued by Cellestia allows us to develop life-saving medicines to treat genetically defined human cancers.
Similarly small molecule mediated targeting of transcription factors allows modulation of Treg cells in lymphoid organs and tissue resident Treg cells. This unique ability to in vivo modulate Treg and T effector cells holds the potential to treat several autoimmune and inflammatory diseases.